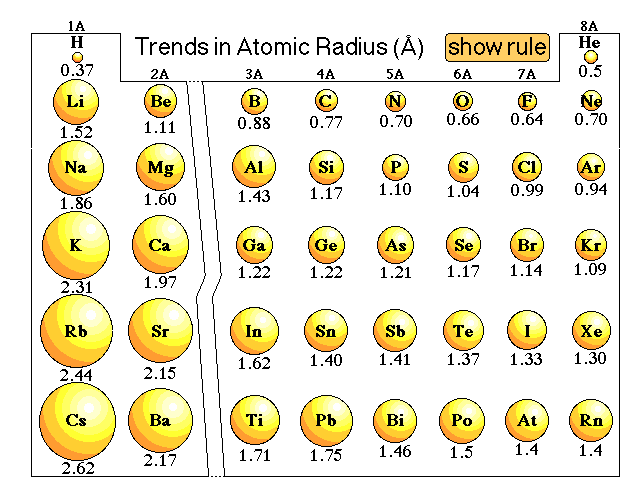
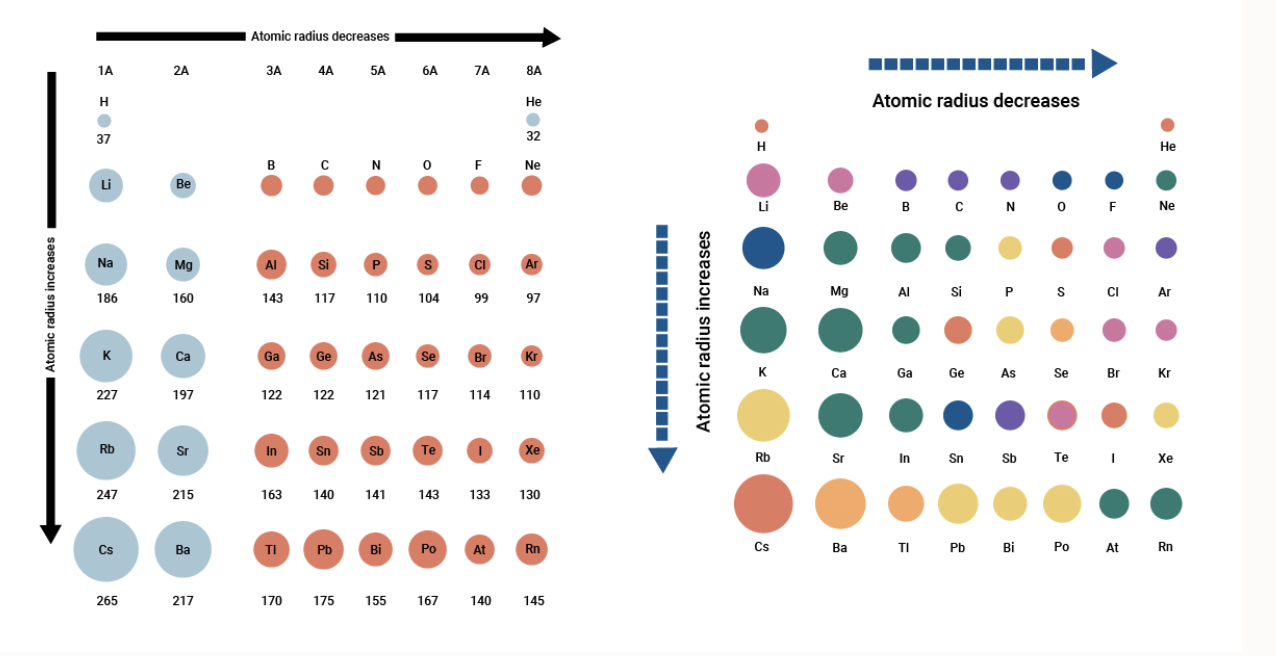
Atomic Radius Measurements of Diatomic Molecules Stock Vector - Illustration of hydrogen, material: 192485256

Gold occurs as face centred cube and it has a density of 19.30 kg dm ^-3 .Calculate atomic radius of gold. (Molar mass of Au = 197 )

Niobium has a density of 8.57 g/cm3 and crystallizes with the body-centered cubic unit cell. Calculate the radius of a niobium atom - Chemistry Stack Exchange
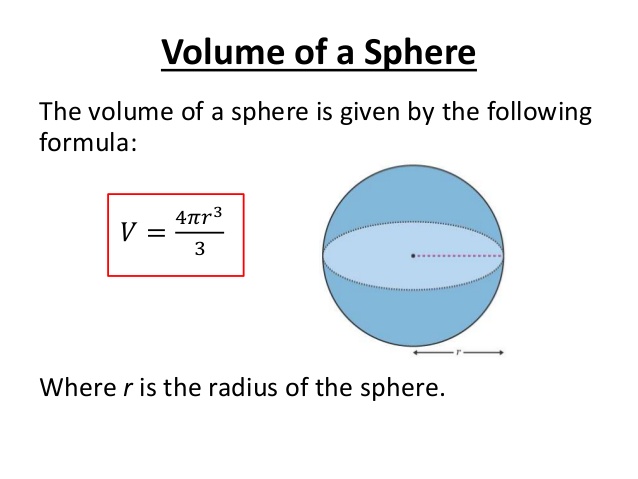
Atomic radius is of order 10^-8 cm and nuclear radius is of order 10^-13. calculate what fraction of atom is occupied by nucleus? | Socratic
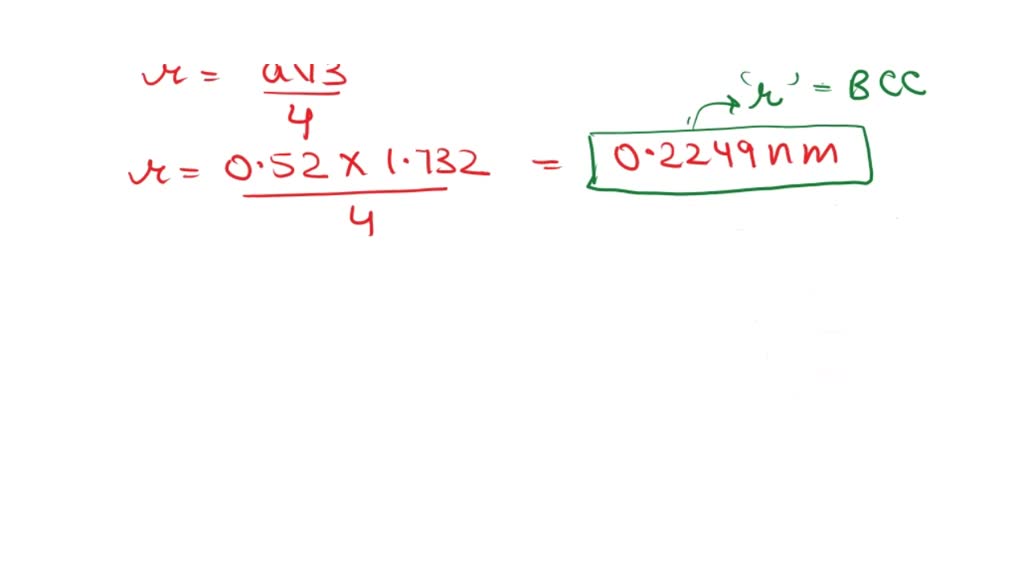
SOLVED: Calculate the atomic radius in cm for the following: (a) BCC metal with a0 = 0.52 nm; and (b) FCC metal with a0 = 3.1 nm

Calculate atomic radius of elementary silver which crystallites in face centered cubic lattice with unit - Brainly.in
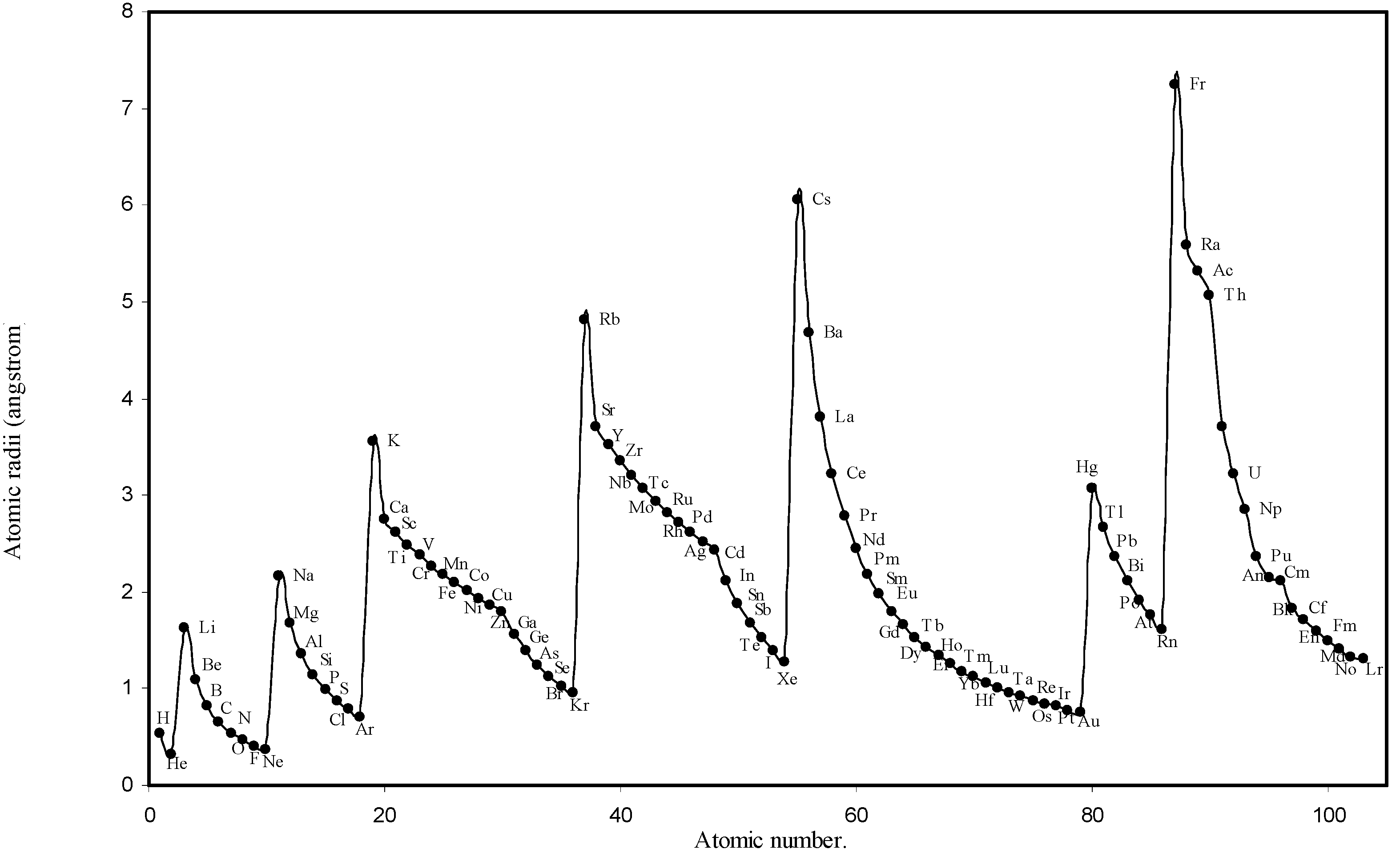
IJMS | Free Full-Text | Theoretical Calculation of Absolute Radii of Atoms and Ions. Part 1. The Atomic Radii