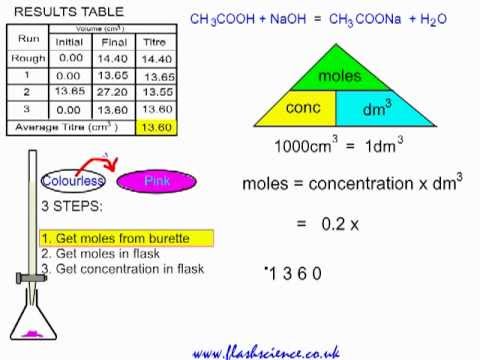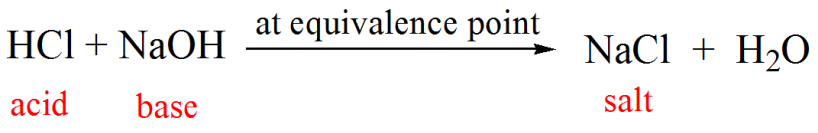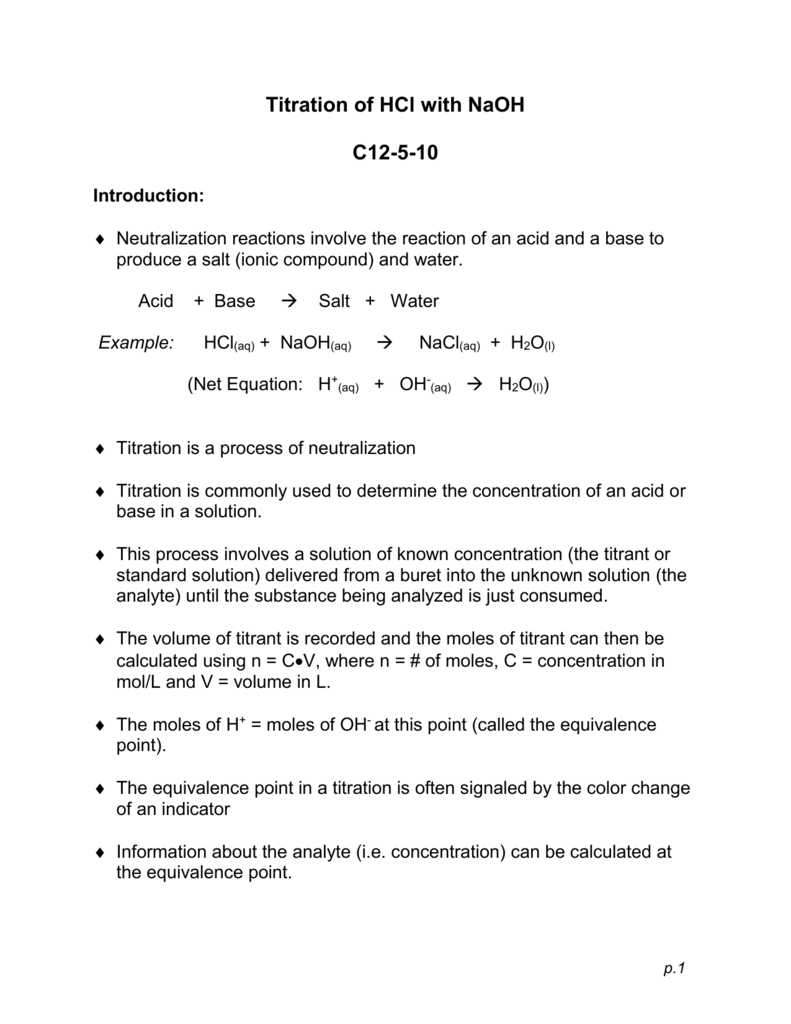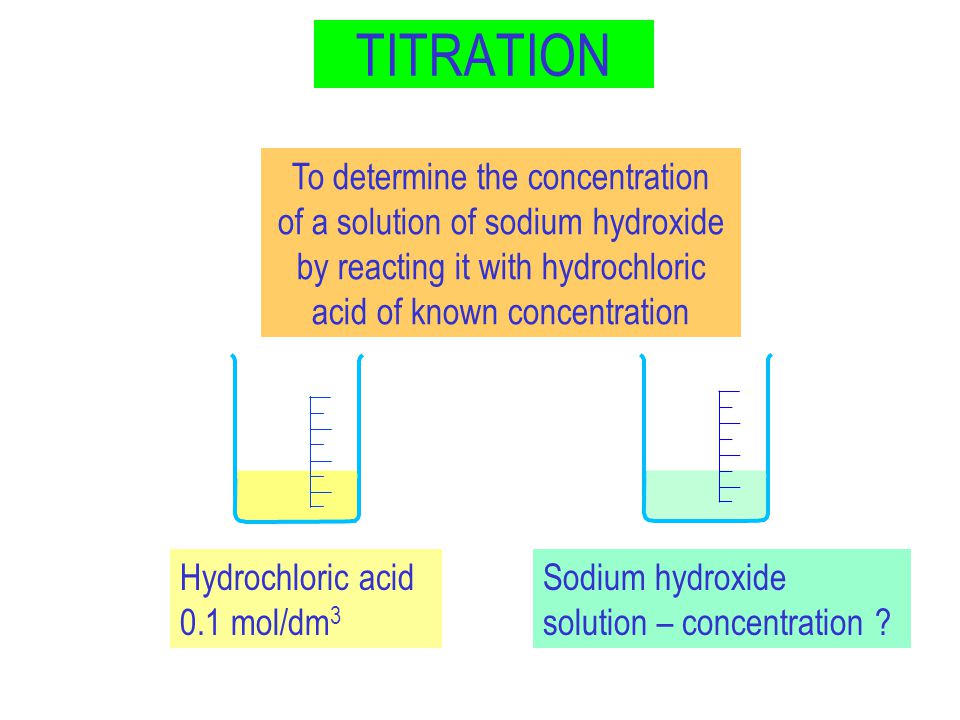
TITRATION Hydrochloric acid 0.1 mol/dm 3 Sodium hydroxide solution – concentration ? To determine the concentration of a solution of sodium hydroxide by. - ppt download
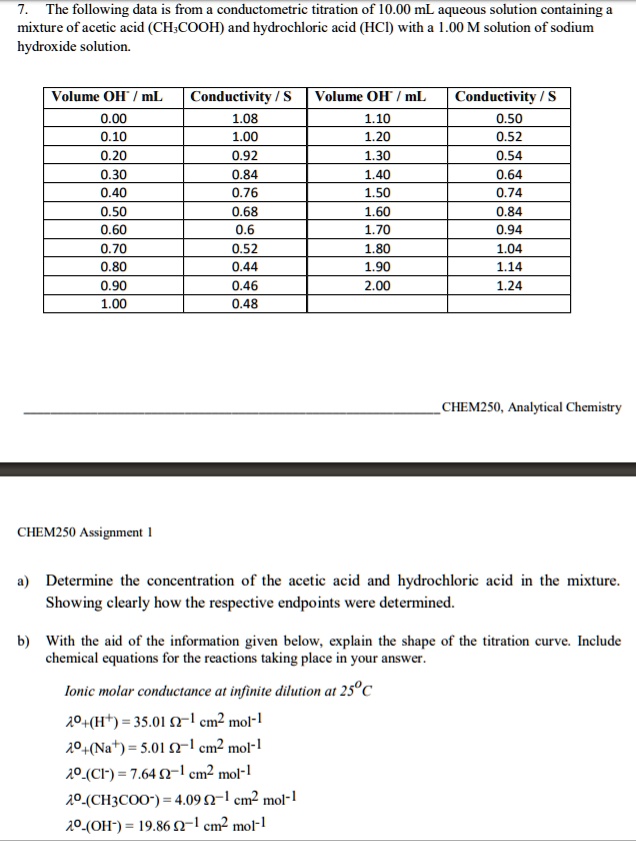
SOLVED: The following data is from conductometric titration of 10.00 mL aqueous solution containing mixture of acetic acid (CHCOOH) and hydrochloric acid (HCI) with 1.00 M solution of sodium hydroxide solution Volume
What volume of 0.1mol/dm3 hydrochloric acid will be required to neutralize 20cm3 of 2.0mol/DM3 sodium hydroxide? - Quora

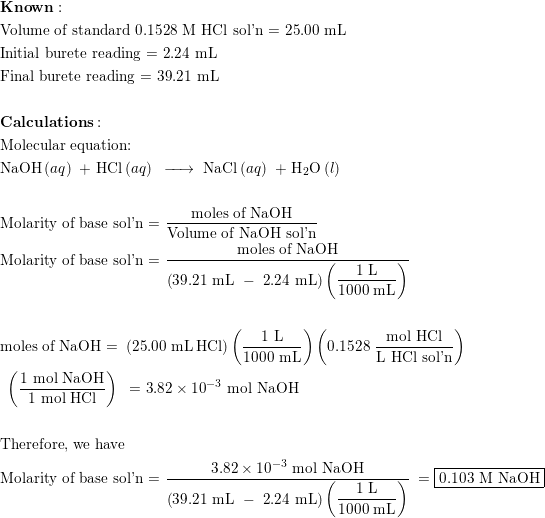
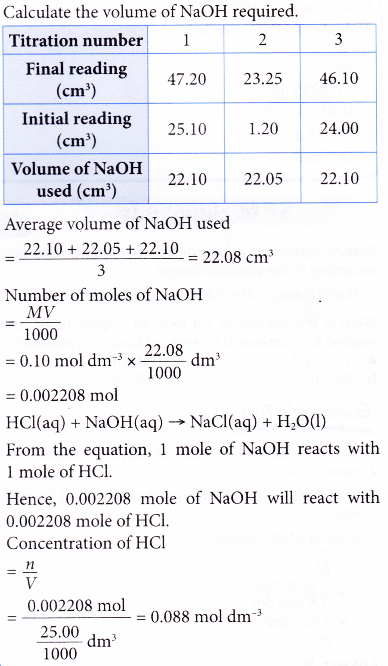
Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.

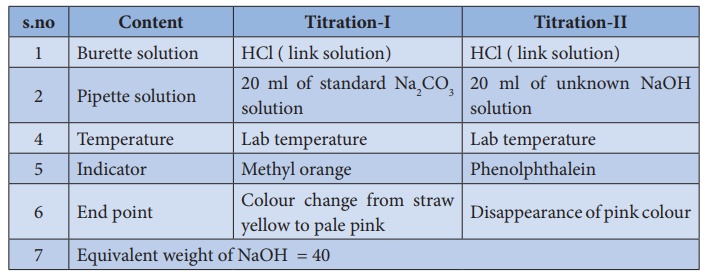
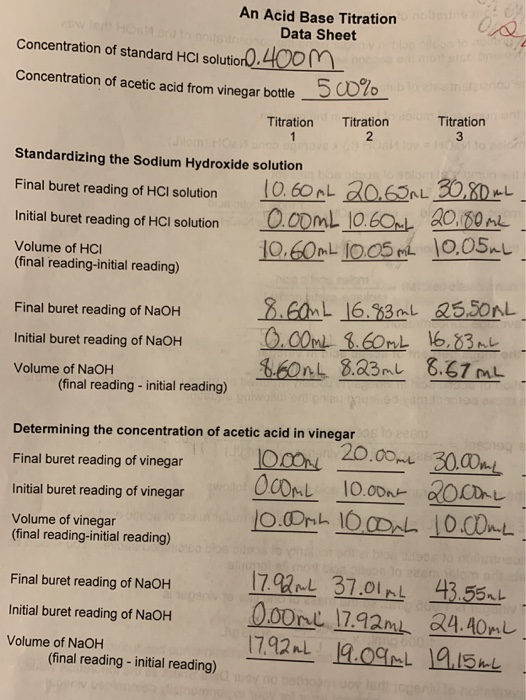
Lab VIII – Titration of Weak (CH3COOH) and Strong (HCL) Acids via Strong Base (NaOH) | nmiller17chem
A student performs a second titration in which the student titrates a 20. mL sample of 0.20 M HCl(aq) with 0.10 M NaOH(aq). How many ml of 0.10 M NaOH(aq) would be

Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.
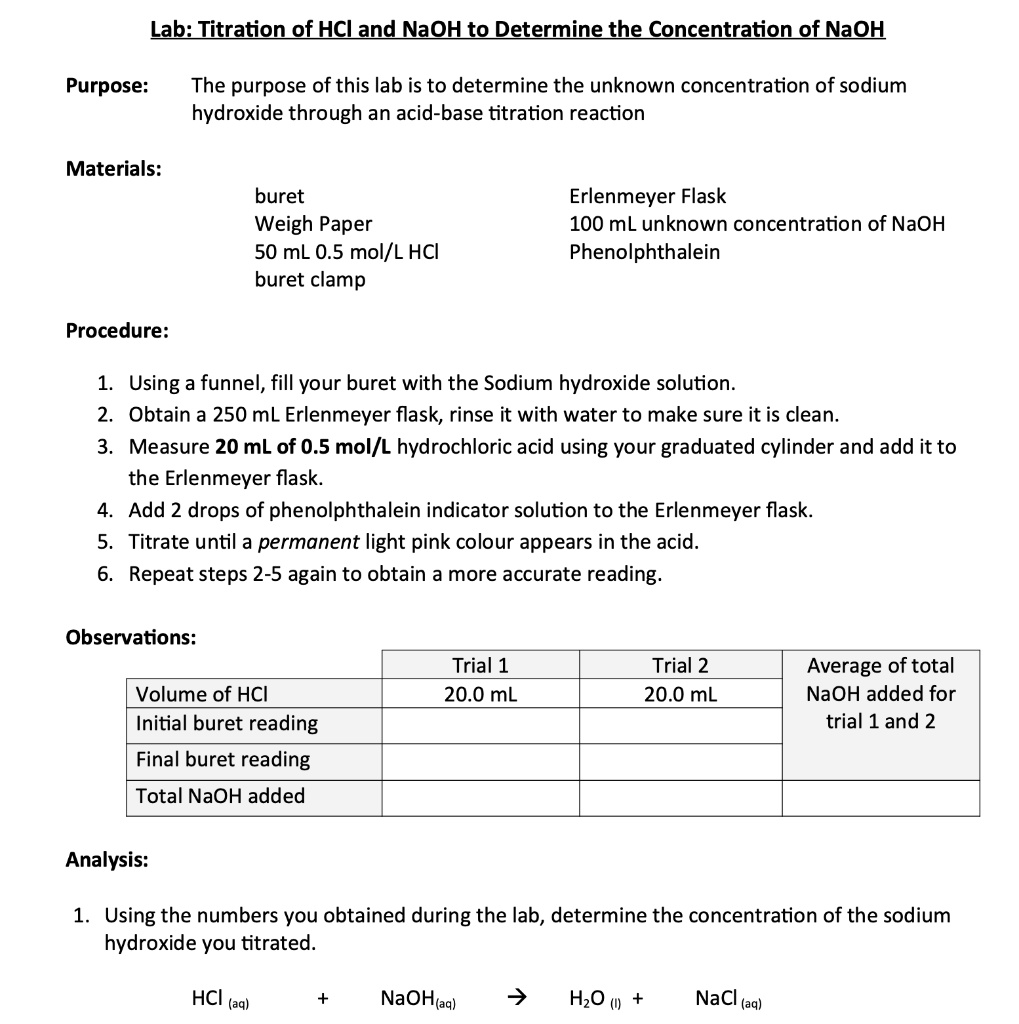
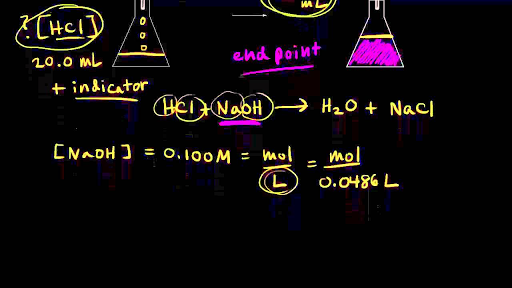
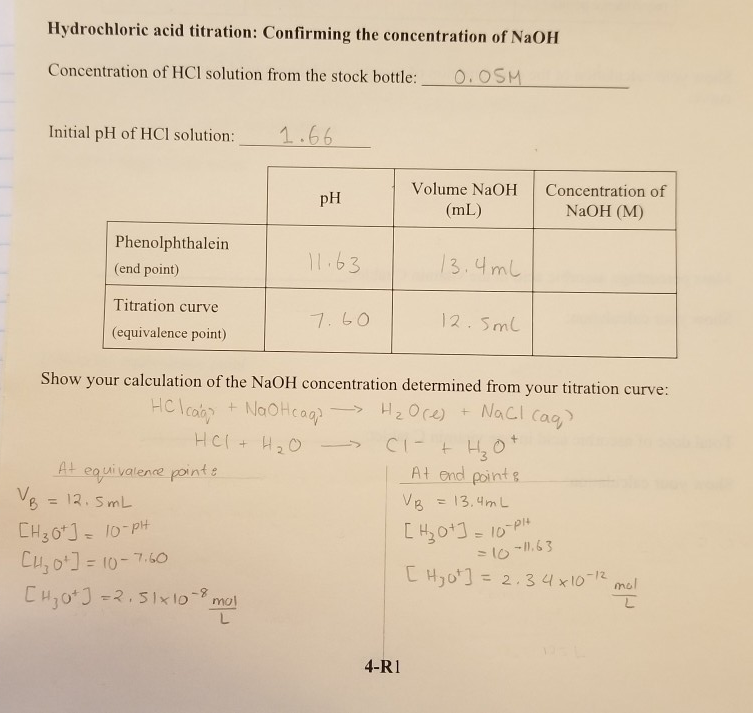
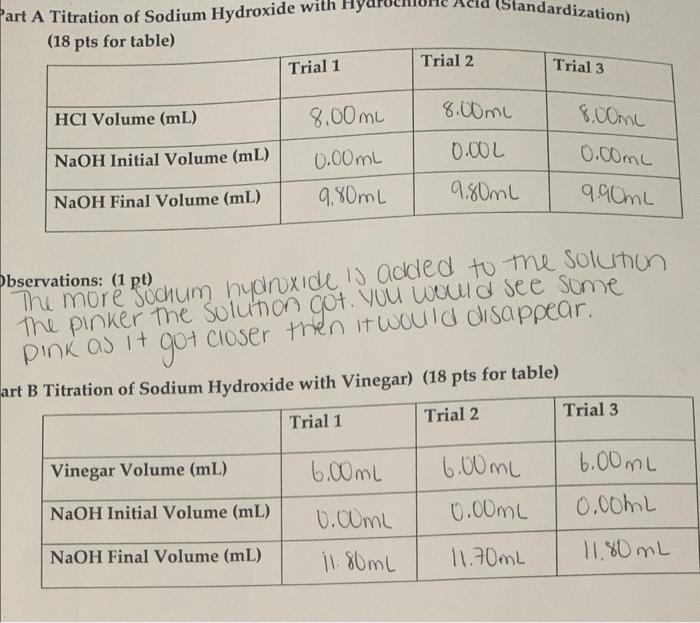
SOLVED: Lab: Titration of HCLand NaOHto Determine the Concentration of NaOH Purpose: The purpose of this lab is to determine the unknown concentration of sodium hydroxide through an acid-base titration reaction Materials: