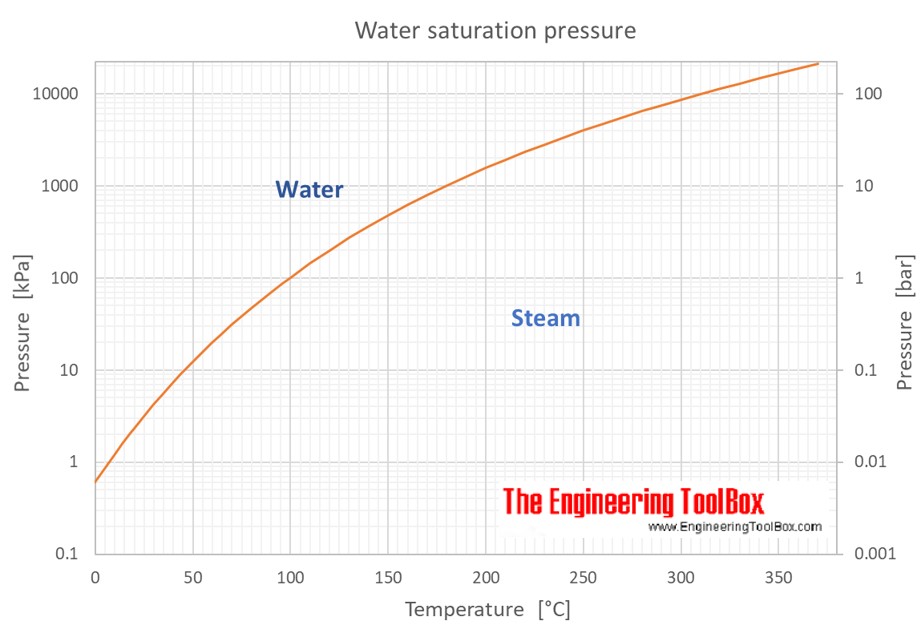
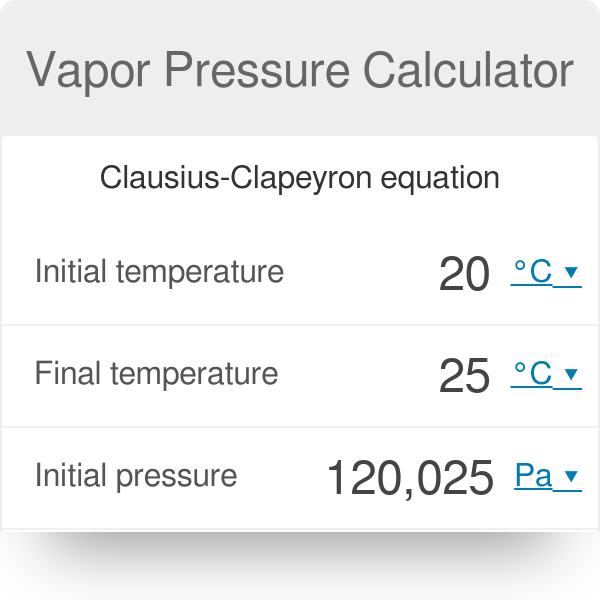
Vapor Pressure Formula & Example | How to Calculate Vapor Pressure - Video & Lesson Transcript | Study.com
Calculate vapour pressure of 0.1M urea soln. Vapour pressure of water at the given temperature is 20 torr. Assume molality and molality to be equal.
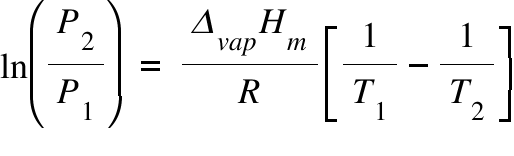
Correlation of Vapor Pressure at Different Temperatures by Clausius Clapeyron Equation Calculator | Calistry

Calculate the vapour pressure of aqueous 0.1 m glucose solution at 300 K temperature, the vapour pressure of water is 0.03 bar at 300 K temperature.