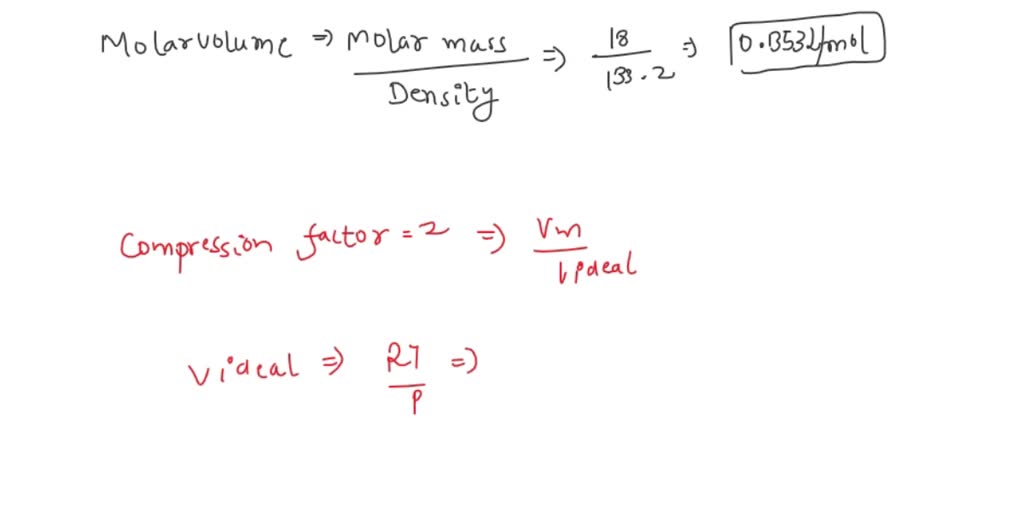
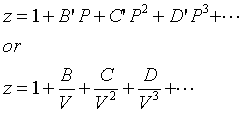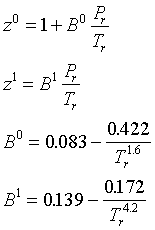The virial form of van der Waal's gas equation is PV = RT (1 + BV + CV^2 + .... ) = RT(1 + B'P + C'P^2 + ....) . The second
SOLVED: The virial equation of state may also be written as an expansion in terms of pressure: Z = 1 + B'p + ... The critical constants for water, H2O, are 218.3

The virial form of van der Waal's gas equation is PV = RT (1 + BV + CV^2 + .... ) = RT(1 + B'P + C'P^2 + ....) . The second

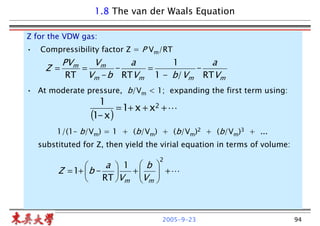
PPT - Advanced Thermodynamics Note 2 Volumetric Properties of Pure Fluids PowerPoint Presentation - ID:756864

The virial form of van der Waal's gas equation is PV = RT (1 + BV + CV^2 + .... ) = RT(1 + B'P + C'P^2 + ....) . The second
![Virial equation is: PV(M)=RT[A+(B)/(V(M))+(C )/(V(M^(2)))+…], where A, B, C, …. are first second,third, … virial coefficent, respectively, For an ideal gas Virial equation is: PV(M)=RT[A+(B)/(V(M))+(C )/(V(M^(2)))+…], where A, B, C, …. are first second,third, … virial coefficent, respectively, For an ideal gas](https://d10lpgp6xz60nq.cloudfront.net/ss/web/298832.jpg)
Virial equation is: PV(M)=RT[A+(B)/(V(M))+(C )/(V(M^(2)))+…], where A, B, C, …. are first second,third, … virial coefficent, respectively, For an ideal gas



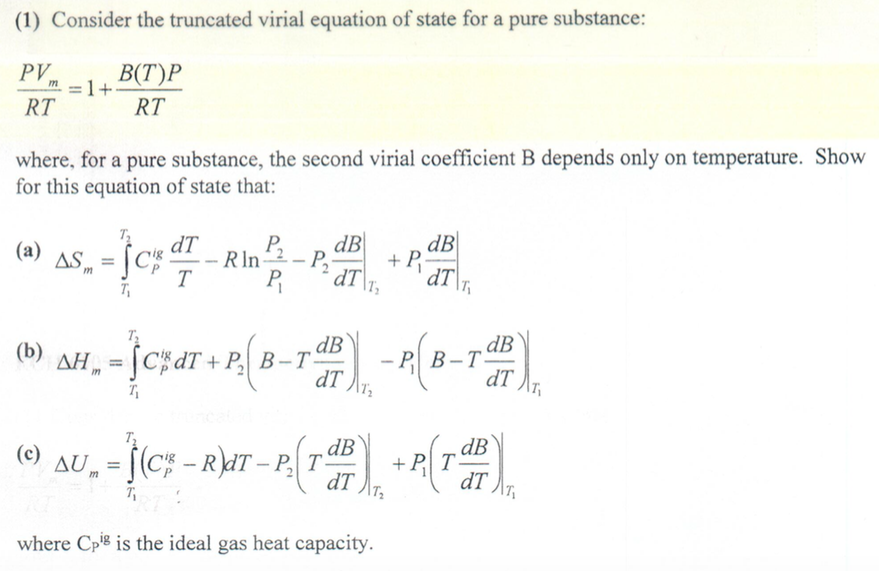
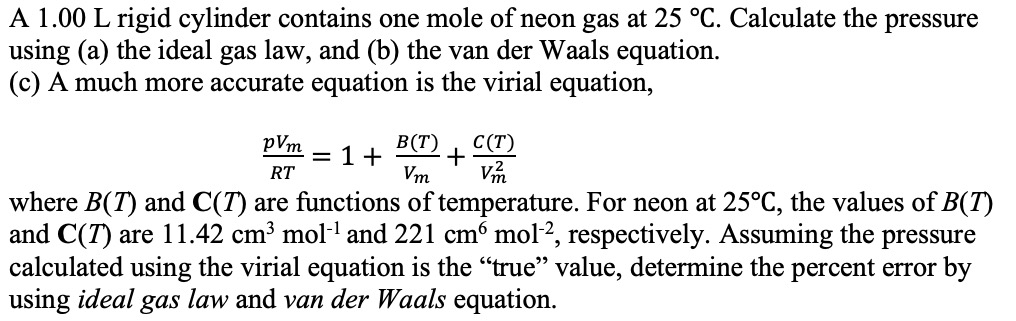
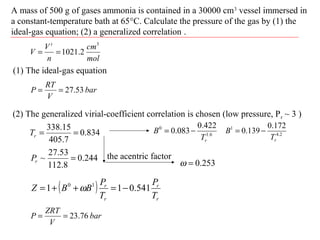

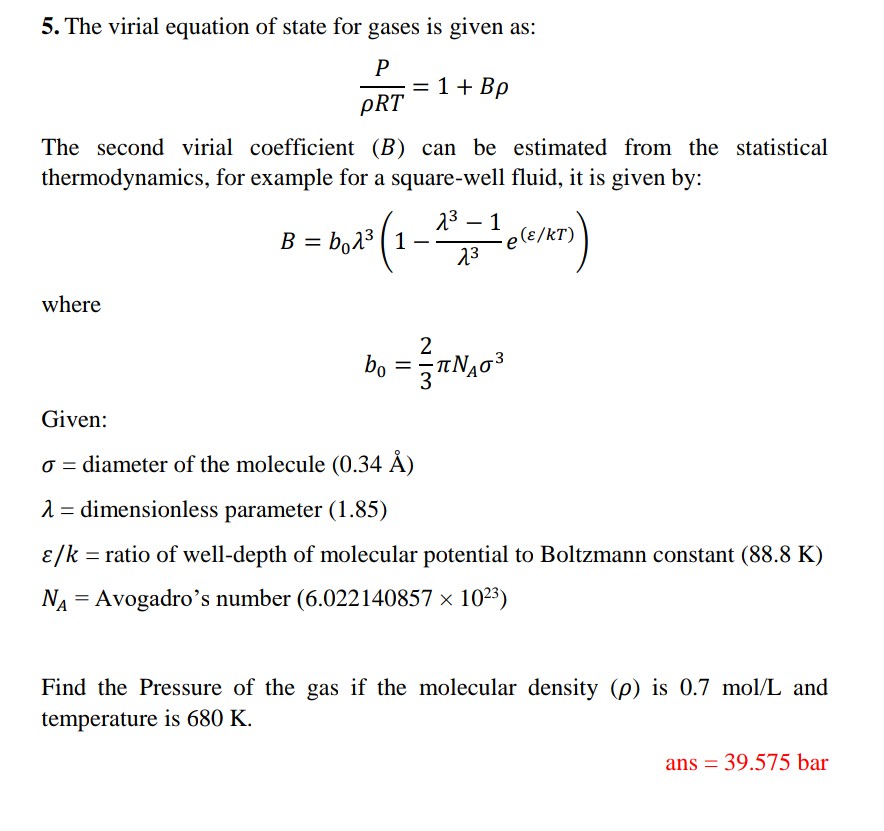
![Solved (a) [5] Suppose a gas obeys the virial equation of | Chegg.com Solved (a) [5] Suppose a gas obeys the virial equation of | Chegg.com](https://media.cheggcdn.com/media/e35/e35166e6-4ef8-480a-937c-7ba4a62e3302/phpM72A7h)